Product Overview for buy demo veenat 400mg tablet
Demo Veenat 400mg Tablet is supplied for institutional procurement and international distribution programs that require consistent documentation, controlled sourcing, and dependable export coordination. Oddway International supports B2B buyers looking to source demo veenat 400mg tablet India for regulated and semi-regulated markets, subject to local regulations and import permissions. Moreover, we align supply planning with distributor timelines to reduce procurement gaps and ensure continuity for hospital tenders and specialty channels.
For buyers evaluating demo veenat 400mg tablet online procurement workflows, we provide a structured quotation and order confirmation process, including batch details, shelf-life information (where available), and export-ready paperwork. Additionally, we support urgent and time-sensitive shipments for authorized programs, including Named Patient Supply (NPS) requests, where permitted.
For broader category navigation and sourcing pathways, many buyers also use our Specialty Pharmacy and Generic Pharmacy Online pages to streamline product discovery and procurement routing.
Therapeutic Category / Drug Classification
Demo Veenat 400mg Tablet belongs to a prescription-only therapeutic segment and is handled as a controlled, documentation-led pharmaceutical product in many jurisdictions. However, classification and import requirements vary by country. Therefore, buyers should confirm local schedules, labeling rules, and any special authorization requirements before placing purchase orders.
Oddway International supports B2B customers with product documentation alignment and communication needed for compliant cross-border sourcing, without making patient-facing or outcome-based claims.
Key Advantages for Importers, Distributors & Hospitals using demo veenat 400mg tablet online
Importers and hospital procurement teams often prioritize supply reliability, clear documentation, and responsive coordination. Consequently, our process focuses on reducing friction from inquiry to dispatch while maintaining traceability. We also help buyers evaluate commercial considerations such as demo veenat 400mg tablet cost and lead times based on destination requirements, shipping mode, and requested brand availability.
- Export-oriented coordination: aligned timelines for distributor restocking, hospital tenders, and institutional demand.
- Documentation support: COA and standard shipping documentation provided as required for import clearance.
- Batch traceability: batch/expiry details shared where available and appropriate.
- Special handling readiness: GDP-aligned packing practices; cold-chain coordination available when applicable to the shipment profile.
- Communication clarity: proactive updates on packing, dispatch, and airway bill details once shipped.
Moreover, we help procurement teams compare commercial parameters such as demo veenat 400mg tablet price across order sizes and delivery terms, without publishing retail pricing or making assumptions that could conflict with local controls.
Wholesale, Export & Named Patient Supply Capabilities for demo veenat 400mg tablet India
Oddway International operates as a trusted Indian pharmaceutical exporter supporting wholesalers, distributors, hospitals, and institutional buyers. We manage wholesale supply and export execution with a focus on compliance, documentation, and shipment integrity. Furthermore, we support urgent NPS shipments for authorized requests, where permitted, including coordination for physician/hospital documentation requirements as per destination rules.
For program-based sourcing and category context, some buyers reference our demo cancer page to align internal procurement mapping and therapeutic portfolio planning.
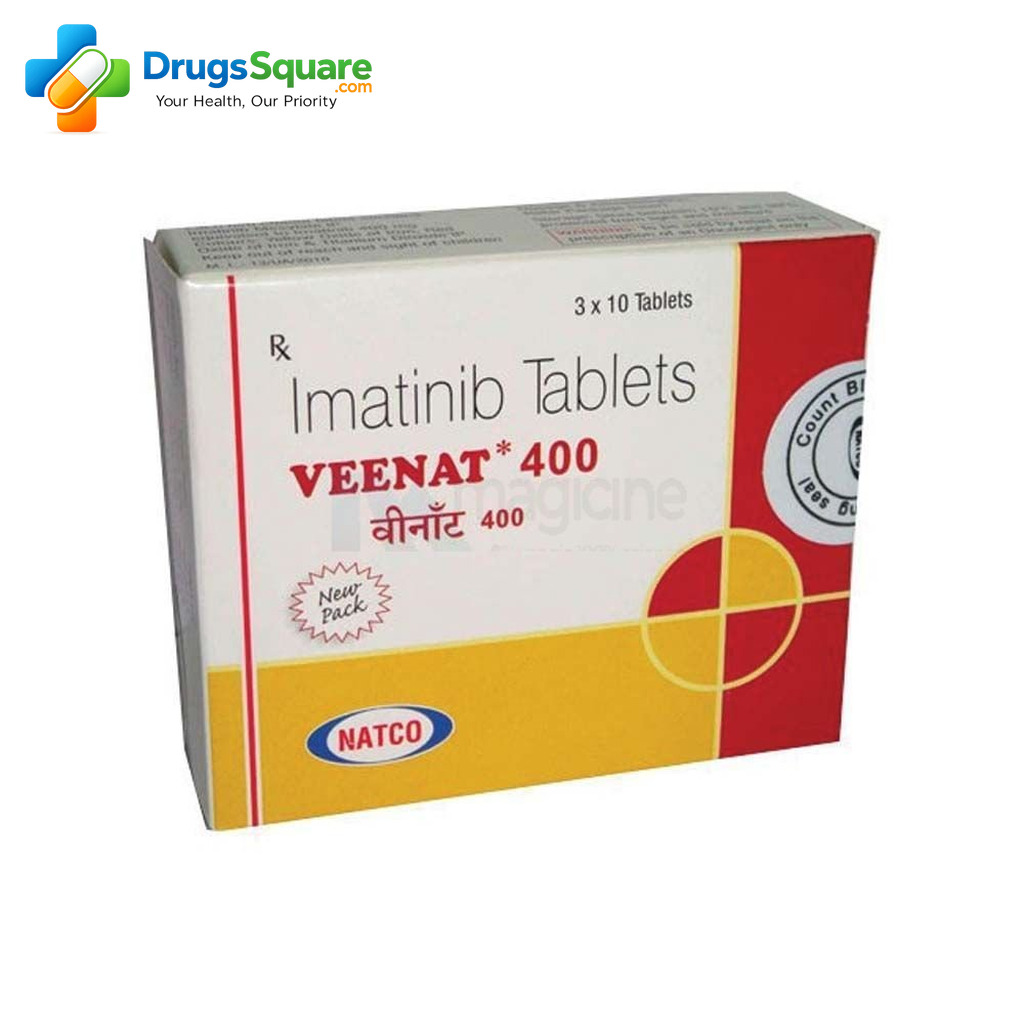
Available Strengths, Packaging & Regulatory Compliance for demo veenat 400mg tablet cost
Demo Veenat is requested commonly in 400 mg strength as a tablet dosage form. Packaging can vary by manufacturer and market channel; therefore, we confirm pack size, labeling language, and serialization needs at quotation stage. Additionally, we support buyer-specific labeling and documentation expectations, subject to manufacturer capability and local regulations.
Regulatory compliance support may include COA, batch details, and other export documents required for clearance. However, final import approval remains the responsibility of the buyer and local authorities. We encourage importers to share destination requirements early so we can align the shipment plan accordingly.
Quality Assurance & Global Standards
We follow a quality-first approach for B2B supply, emphasizing traceability and documentation readiness. Consequently, we coordinate procurement from reputable channels and support standard quality documents such as COA and, where requested, MSDS/SDS. Moreover, we apply GDP-aligned packing practices and help buyers plan suitable transit conditions based on route and climate considerations.
Where required, we can support third-party inspection coordination and pre-dispatch checks, subject to feasibility and timelines. This helps institutional buyers maintain internal compliance and supplier qualification workflows.
Why Source from Oddway International for buy demo veenat 400mg tablet
Oddway International supports global buyers with structured export operations, responsive communication, and experience shipping pharmaceuticals from India to multiple regions. Additionally, we understand the practical needs of procurement teams—clear proforma invoices, consistent updates, and accurate documentation. As a result, distributors and hospitals can reduce delays at customs and maintain predictable receiving schedules.
We support both regulated and semi-regulated markets, subject to local regulations. Moreover, we can coordinate special handling and urgent dispatch planning when timelines are critical.
International Logistics & Documentation Support
We assist with end-to-end export logistics, including packing lists, commercial invoices, and courier or air cargo coordination based on destination and urgency. Furthermore, we share shipment tracking and dispatch confirmations promptly. Documentation support can include COA and MSDS/SDS when requested, as well as GDP-aligned packing practices to help protect product integrity during transit.
Consequently, buyers can streamline internal receiving, minimize clearance queries, and maintain reliable procurement cycles for institutional supply, wholesale distribution, and NPS channels where permitted.


Samuel K. –
Clear communication from quotation to dispatch. Documents were shared in advance so our clearing agent had time to review everything.
Mariana Lopes –
We had a tight internal approval timeline and Oddway kept the paperwork organized. There was one small correction needed on the consignee format, and they fixed it quickly.
Hassan A. –
Professional handling overall. The team confirmed batch details before shipping and provided regular updates until delivery. This helped us plan receiving and internal QA checks.
Elena Petrova –
Good coordination with freight and prompt responses on email. Lead time matched what was promised, and the shipment file was complete for our records.
Michael Osei –
We’ve worked with multiple exporters, and Oddway stands out for follow-through. They confirmed requirements early, shared scanned documents before couriering originals, and stayed available during customs clearance questions.
Daniel M. –
Clear communication from quotation to dispatch. Documents were shared quickly and the shipment updates were consistent, which helped our receiving team plan properly.
Mariana Lopes –
We had a few destination-specific questions and they handled them patiently. Packing list and invoice matched exactly, and tracking was provided the same day it shipped.
Ahmed El-Sayed –
Good turnaround on the proforma and confirmation. They coordinated the export paperwork well and kept us informed when the airline schedule changed. Overall, reliable partner for repeat procurement.
Sophie R. –
Our order required careful documentation review, and Oddway was responsive on email and WhatsApp. The shipment arrived within the expected window and the batch details were shared in advance.
Peter Ndlovu –
We’ve placed multiple orders and the experience has been consistent. Communication is straightforward, and the dispatch process is well organized. It saves time for our team when documents are correct the first time.